Technology Research & Development (TR&D) Projects
The Hyperpolarized MRI Technology Resource Center is based on 3 Technology Research & Development (TR&D) projects and its development is driven in a push-pull manner by the Collaborative Projects. Below are information on each of the TR&D projects and a summary of the current Collaborative Projects.
TR&D1 Goals:
- Clinical Polarizer Instrumentation Development and Testing
- Preclinical Polarizer & RF Technology Development
- New Instrumentation and Technology for clinical translation of HP 13C MRI
TR&D2 Goals:
- Development and Optimization of HP Probes and Methods
- Identification of Realistic Preclinical Models and Optimization of Methods for Validation of Robust Quantitative HP 13C MR
- Validation of HP Metabolic Imaging Findings via Correlative Pathologic, Molecular and Multimodal Imaging Studies
TR&D3 Goals:
- Novel High-resolution and Robust HP 13C MR Acquisition Technology Development
- Specialized Analysis Methodologies to Enable Accurate and Reliable Interpretation of HP data
- Data Management and Infrastructure for Visualization, Integration, and Sharing
TR&D2 Led By:
TR&D3 Led By:
TR&D Project 1: Technology Development for Polarizer and Detector Instrumentation
Leads:
Daniel Vigneron, PhD
TR&D1 Project Leader
[email protected]
(415) 476-3343
Jeremy Gordon, PhD
TR&D1 Project Leader
[email protected]
(415) 476-9786
Michael Ohliger, MD, PhD
TR&D1 Clinical Lead
Abdominal & Liver Radiologist
Update from 2023 Workshop:
Description:
Hyperpolarized MRI using Dynamic Nuclear Polarization (DNP) is a powerful new imaging technique that uses specialized instrumentation to provide MR signal enhancement of 10,000-100,000 fold for 13C labeled compounds. To advance current hyperpolarized carbon-13 methodology, major advances in DNP instrumentation, DNP methods, and MR acquisition techniques are required to increase the applicability, reliability, and information content of this emerging imaging technology.
This Technology Resource & Development (TR&D1) project is carried out by a multidisciplinary team with extensive expertise in basic NMR science, mechanical, electrical and instrumentation design, bioengineering, and DNP physics and engineering. In addition to this expertise, we have extensive facilities including mechanical and electronics shops, multiple NMR systems, MR scanners, and research DNP polarizers.
The methods developed in this TR&D are disseminated to the Collaborative and Service Project investigators and to the broader HP research community via the center website, publications and workshops. In conjunction with the Collaborative Project investigators and driven by the needs of their research projects, this Technology Research and Development (TR&D1) project seeks to:
- Develop and investigate new DNP polarizer hardware and techniques and establishes SOPs for testing and regulatory approval.
- Develop specialized hardware for in vivo preclinical HP 13C MRI in animal models, including new polarizer technologies, sample delivery methods (including electromagnets), and RF coils.
- Investigate new instrumentation and develop specialized hardware & methods for clinical translation of HP 13C MRI, with a focus on “patient-centered” and ergonomic coil array designs that eliminate the need to swap coils and seamlessly integrates HP 13C MRI into a multi-parametric exam.
Dissemination & Training:
TR&D Project 2: Development of Novel Hyperpolarized MR Molecular Imaging Probes Tested in Realistic Preclinical Models and Correlative Science Studies
Leads:
John Kurhanewicz, PhD
TR&D2 Project Leader
[email protected]
(415) 514-9711
Renuka Sriram, PhD
TR&D2 Project Leader
[email protected]
(415) 514-4874
Robert Flavell, MD, PhD
TR&D2 Clinical Lead
Molecular Imaging and Therapeutics
Update from 2023 Workshop:
Description:
The peer-reviewed and funded Collaborative Projects and the Service Projects from leading hyperpolarized MR sites are the scientific driving forces behind HMTRC developments which address the need for improved hyperpolarized (HP) probes and techniques, and more realistic pre-clinical models of human disease.
This Technology Research and Development (TR&D2) project takes on innovative directions focused on increasing HP 13C probe sensitivity, in vivo performance, and clinical translatability through the development/optimization of HP probes, probe formulations and polarization approaches. The main deliverables of this project are the dissemination of optimized HP probe preparations and methods, new NMR-compatible cell and tissue culture bioreactor
systems and preclinical GEM and PDX models optimized for HP 13C MR studies, correlative pathology, molecular and synergistic imaging approaches and protocols, and the associated dissemination and training for the biomedical community.
This project takes advantage of our extensive experience in combining hyperpolarized MR techniques to increase HP 13C probe sensitivity, in vivo performance, and clinical translatability through the development/optimization of HP probes, probe formulations and polarization approaches. In specific aim 1, we are developing and optimizing HP probes and methods for HP 13C MR studies. As part of specific aim 2, we are identifying realistic preclinical models and optimizing methods for validation of robust quantitative HP 13C MRI. In specific aim 3, we are developing specialized procedures to provide correlative pathologic, molecular, and imaging data vital for understanding and validating HP 13C MR findings.
Dissemination & Training:
TR&D Project 3: Acquisition and Analysis Methods for Hyperpolarized MR Data
Leads:
Duan Xu, PhD
TR&D3 Project Leader
[email protected]
(415) 514-4455
Peder Larson, PhD
TR&D3 Project Leader
[email protected]
(415) 514-4876
Javier Villanueva-Meyer, MD
TR&D3 Clinical Lead
Neuroradiology
Update from 2023 Workshop:
Description:
Specialized fast acquisitions are a necessity for HP MRI studies by the Collaborative Projects (CP) and Service Projects (SP), dictating that data reconstruction and analysis software platforms must be flexible and expansive, covering a variety of coil geometries, k-space trajectories, vascular delivery and molecular kinetics. The analysis of HP MRI data also requires specialized methods that account for the bolus kinetics and vascular effects as well as specialized evaluation approaches since ground truth data is typically not available for comparison. The challenges remaining for HP acquisition and analysis include quantifying kinetics, heterogeneity of strategies across the user community, and how to leverage data-driven, machine learning methods for HP MRI. To provide next generation HP technology as well as to enable multi-site data harmonization and large-scale data analysis, the Main goals of this Technology Research and Development (TR&D3) project: Acquisition and Analysis Methods for Hyperpolarized MR Data are to: 1) Develop next-generation pulse sequences, reconstruction methods, and analysis methods for HP-13C MRI to provide improved spatio-temporal resolution, coverage, and sensitivity to metabolism. 2) Create standardized methods (phantoms and protocols) to enable multi-site comparison and trials. 3) Provide advanced HP tools through open-source software that will support next generation acquisition and analysis innovations throughout the CPs, SPs and the HP MRI community. 4) Develop HP MRI analyses that are integrated with other imaging and clinical data.
The resulting techniques will be disseminated through free, open-source software to service projects and the general scientific community using our established software frameworks (SIVIC, hyperpolarized-mri-toolbox) and through the center website.
Dissemination & Training:
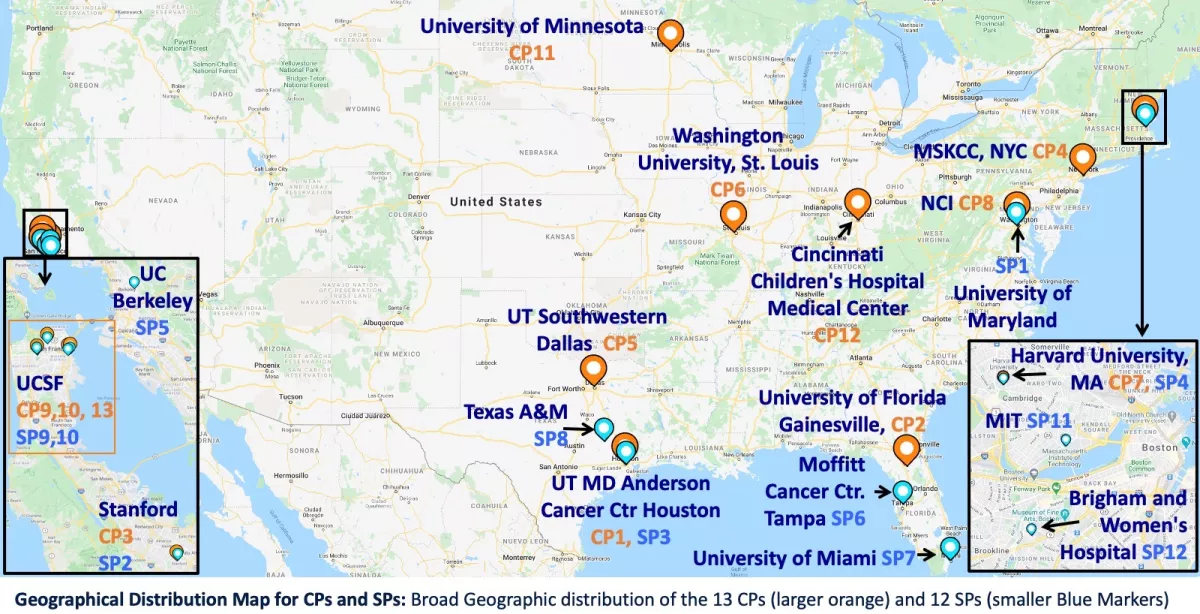
Collaborative Projects & Service Projects
Through our Collaborative Projects we develop technology resources that provide significant biomedical impact while driving the technology developed at the HMTRC in a push-pull manner. Our Service Projects work with and benifit from the expertise in the center for consultation, data interpretation, software access, technical support, or instrumentation.
Collaborative Projects
Project 1: Clinical and Preclinical HP C-13 MRI Research at MDACC UT Houston
PI: James Bankson PhD, University of Texas MD Anderson Cancer Center (MDACC), Houston, TX
Project Goal: Current methods for assessing prostate cancer, the second most common cause of cancer death in men, do not adequately distinguish between aggressive and indolent disease. Over- or under-treatment due to suboptimal diagnostics can lead to unnecessary loss of life or devastating decline in quality of life. New methods that better assess disease aggressiveness could substantially reduce long-term costs and improve the quality of life of men affected by prostate cancer. This ongoing CP includes both preclinical and clinical HP MRI research that interacts with all 3 TRDs and is funded by 3 grants that continue and expand this collaborative project. R01CA211150 funds novel, multi-site HP 13C-pyruvate studies at both MDACC and UCSF leveraging the acquisition expertise at UCSF with the large patient population and analysis expertise at MDACC. UCSF HP SPINlab polarization and HP MRI acquisition methods were successfully transferred to, implemented, and tested at MDACC to enable first HP C-13 MRI studies to be conducted in Houston last year. The P01 project 3 “Inhibiting Oxidative Phosphorylation in Pancreatic Cancer” (PI Pant) is a new direction for this collaboration and the HMTRC will provide (push) new preclinical developments including specialized methods for the new Polarize SpinAligner 6.7T polarizer that Dr. Bankson has also ordered for preclinical pancreas MRI studies. Also, the HMTRC will disseminate information on human pancreas studies that have recently been started at UCSF including coils, sequences, protocols and sample anonymized data. The new R21 on HP studies of the brain also represents a new direction that will benefit from the technologies developed in this HMTRC driven by other past & current CPs focused on brain clinical research. This R21 project also will pull the center to develop and disseminate with the appropriate training, new technologies for optimal HP C-13 MRI of the brain with specialized MR acquisition methods and collaboratively developed analysis methods that will be integrated into the free, open-source software developed by TRD3.
Project 2: Preclinical HP C-13 MR Studies of Metabolic Diseases
PI: Charlie Khemtong PhD, University of Florida
Project Description: This recently funded new CP project led by Dr. Khemtong (previously at UTSW) and with his colleague Dr. Merritt at UF, investigates preclinical liver & cardiac metabolism and diabetes studies with HP C-13 utilizing TRD improvements for HP MR with DHAc & other new 13C probes for tissue pH and energy metabolism utilizing technology developed through this NCBIB. Abnormal metabolism is associated with a number of metabolic diseases such as cancer, diabetes, and cardiovascular diseases. Important markers for the altered metabolic states include changes in fuel preferences and loss of acidity regulations in tissues. To date, routine assessments of energy metabolism and acidity in viable tissues remain a challenge. Sensitive imaging modalities that can provide accurate characterization of these important biomarkers are therefore highly desirable. This project aims to establish a series of hyperpolarized (HP) 13C-enriched molecules that can be activated by an endogenous enzyme esterase to produce both pH and metabolic imaging probes in tissues. Successful development of these HP 13C-enriched compounds will allow for tissue acidity and metabolic information to be simultaneously characterized following an injection of a single imaging probe. Molecular candidates for these applications must be chemically stable, can be highly polarzed by dynamic nuclear polarization (DNP), have long 13C T1’s, and be highly sensitive to esterase hydrolysis. Once hydrolyzed, the molecules must quickly decompose to form a pair of pH imaging probes, HP 13CCO2 and HP H13CO3-, as well as a HP 13C-enriched metabolic substrate such as 13C-pyruvate. This project aims to investigate a series of ethyl alkyl mixed anhydride carbonate compounds with 13C enrichments at both the carbonate and carboxyl carbons. These carbon centers have long T1 making them suitable for HP 13C imaging applications. Moreover, these small organic molecules are expected to be efficiently dDNP polarized with significantly improved 13C MR imaging sensitivity. Also, the ester functional group in these mixed anhydrides is expected to be highly susceptible to hydrolysis by esterase. Once injected into the circulation, it is expected that these HP 13C-mixed anhydride carbonates will be rapidly hydrolyzed by esterase producing monoacyl carbonate molecules. These intermediates are expected to decompose, producing HP 13CO2 and a HP 13C-enriched metabolic substrate for pH measurement and metabolism analysis in tissues, respectively.
Project 3: Imaging Biomarkers for Glioma Treatment Response
PI: Daniel Spielman PhD, Stanford University
Project Description: The overall goal of this CP project is to assess the potential synergy of combining information on glucose uptake, as provided by FDG-PET, and pyruvate metabolism, as provided by HP 13C, for brain tumor characterization & assessment of response.
Significance: Alteration of metabolism so as to favor a preponderance of glycolysis (GLY) relative to oxidative phosphorylation (OXPHOS) is considered a hallmark of cancer. Known originally as the Warburg effect, and now considered part of the larger concept of metabolic reprogramming, these cellular changes represent a way for cancer tissue to support rapid proliferation by preserving carbon skeletons for biomass production. Understanding the mechanisms that underlie this metabolic shift is an active area of research. In the context of therapeutics, insights from recent studies provide strong support that this reprogramming phenotype is necessary and sufficient to support the cancer process, thus providing a basis for highly novel therapeutic strategies in which either blocking or reversing metabolic reprogramming is the goal. Furthermore, malignant gliomas, highly glycolytic cancers exceedingly resistant to conventional treatments, seem particularly suited to approaches that can subvert this phenotype, and a crucial obstacle to moving such therapies to clinic has been the inability to reliably measure in vivo response to such metabolic therapies. The scientific premise of this proposal is that hyperpolarized 13C (HP13C) magnetic resonance imaging (MRI) offers great promise in fulfilling this clinical need. Pyruvate (Pyr), located at a crucial juncture in the brain glucose metabolic pathway where it can be either reduced to lactate (Lac) or converted to acetyl CoA + CO2, which is then converted to bicarbonate (Bic), has the potential to be used as a HP13C surrogate marker of the balance between GLY and OXPHOS. Following the bolus injection of HP [1-13C]Pyr, the observed 13C-Lac/13C-Bic (Lac/Bic) ratios could be useful as a quantitative biomarker of a changing balance between these two metabolic processes, thus providing key information on glucose’s metabolic fate complementary to the uptake information provided by more commonly available 18F-fluoro-deoxy-glucose positron emission tomography (FDG-PET). This CP proposes to add simultaneous FDG-PET/HP13C/MRI measurements to an upcoming Phase II clinical trial of malignant glioma treated with BPM31510 (Berg LLC), a nano-suspension of Coenzyme Q10 showing high accumulation in cancer cell mitochondria and having marked antitumor activity in multiple in vivo models (both alone and in combination with chemotherapeutic agents) with in vitro evidence strongly suggesting the effect is mediated via increasing OXPHOS (i.e., reversing the Warburg effect). The overall goal of this CP is to assess the potential synergy of combining information on glucose uptake, as provided by FDG-PET, and glucose metabolism, as provided by HP 13C MRI, for tumor characterization, assessment of therapeutic response, and prediction of patient outcome.
Project 4: Exploring Cancer Metabolism with HP C-13 MRI
PI: Kayvan Keshari PhD, Memorial Sloan Kettering Cancer Center
Project Description: This ongoing and expanded CP funded by 3 recently funded R01’s focuses on both preclinical HP research and translational patient studies.
Significance: Prostate cancers, currently the most common cancer in men, demonstrate a tremendous range of biologic diversity. Clinical assessments of response to non-surgical therapy are often inadequate because, as studies have shown, they lead to inaccuracies when they rely upon serum prostate specific antigen (PSA) levels reaching a nadir, or upon the histological confirmation of cancer using transrectal ultrasound guided biopsies. When progressing to metastatic cancer, typically after androgen deprivation therapy, castrate resistant prostate cancer (CRPC) results in bone lesions in more than 90% of cases. There remains a critical clinical need for greater sensitivity and specificity in molecular imaging biomarkers of prostate cancer presence and response to novel therapeutics. The over-arching goal of this proposed research is to translate a new MRI technology, hyperpolarized (HP) MR, to the clinic in the setting of large-field-of-view (FOV) MRI, which utilizes both the agents HP [1-13C]pyruvate and [2-13C]pyruvate in metastatic prostate cancer patients. Prostate cancer demonstrates tremendous biologic diversity, and there is an urgent need to develop more sensitive and specific imaging biomarkers to characterize the disease. We aim to develop a large-body transmit/receive system and pulse sequences for large FOV and apply them to a cohort of prostate cancer patients: this work will aid in future patient-specific treatment planning, facilitate earlier assessment of response to therapy, and facilitate the development of novel experimental strategies for cancer treatment. The goal of R01CA248364 “Leveraging fructose transport to create a privileged substrate to selectively fuel T cells” is to explore the metabolism of T cells in order to develop metabolic imaging approaches with hyperpolarized (HP) MR. It has been shown that T cell therapies are hindered by a dearth of metabolic precursors in the tumor microenvironment. This preclinical research aims to better understand T cell metabolism using novel tools and models and to develop strategies to overcome metabolic limitations. This project aims to enhance the glycolytic metabolism of T cells by giving them a unique endogenous substrate for their metabolism, fructose, which is present in our diet but not normally metabolized by a wide range of cancers. This work seeks to advance the treatment of a range of solid tumors and develop non-invasive metabolic biomarkers for cancer immunotherapy. The overarching goal of NIH R01CA252037 “Visualizing oxidative stress using hyperpolarized magnetic resonance” is to conduct novel research for imaging oxidative stress using hyperpolarized dehydroascorbate (HP DHA) MRI. Oxidative stress is implicated in the pathogenesis of many diseases, including neurodegeneration and brain tumors, and there is an urgent need to develop more sensitive and specific imaging approaches to characterize it. We aim to develop HP DHA MRI and apply it to preclinical models of oxidative stress in the brain and brain tumors as well as optimize it for translation into humans. This work will facilitate future patient-specific treatment planning, as well as earlier assessment of response to therapy and the development of novel experimental strategies for cancer treatment.
Project 5: Studying Cerebral Energy Metabolism Following Traumatic Brain Injury with HP C-13 MRI
PI: Jae Mo Park PhD, University of Texas Southwestern Medical Center, Dallas, TX
Project Description: This new project leverages the extensive past HP C-13 MR experience of these investigators with training and technology dissemination from this NCBIB.
Significance: Traumatic Brain Injury HP C-13 research will drive the TRDs to develop improved monitoring of cerebral energy metabolism and neurotransmitter synthesis with both C-1 and C-2 pyruvate HP MRI techniques for this new CP. Traumatic brain injury (TBI) is a leading cause of death and disability among children and young adults in the United States. Each year an estimated 1.5 million Americans sustain a TBI; 230,000 people are hospitalized. A major challenge of treating traumatic brain injury (TBI) patients is the simultaneously occurring complex secondary injury processes following the primary injury. The secondary events such as cerebral hyperglycolysis and mitochondrial failure develop over minutes to months after the primary injury, providing a potential window of opportunity for therapeutic intervention. Given early, this intervention may prevent or reduce secondary brain damage, directly impacting long-term patient outcome. Therefore, the noninvasive detection and characterization of pathophysiology in TBI patients during the acute and early sub-acute stages, will have critical clinical implications for the early diagnosis of individuals with the highest risk of poor neurological outcomes and will be vital for identifying and developing effective therapies. While a number of pathological alternations in TBI are potential biomarkers, no current clinical imaging modalities are sensitive enough to be routinely used to detect the details of metabolic shifts in brain sub-regions with secondary injury. Magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized 13C-labeled substrates provides unique noninvasive measurements of critical in vivo dynamic metabolic processes. In particular, pyruvate occupies a key nodal point in cerebral energy metabolism, among the fates of [1-13C]pyruvate are reduction to lactate as the end product of glycolysis, conversion in mitochondria to form acetyl-CoA and CO2 via pyruvate dehydrogenase (PDH) flux or anaplerotic pyruvate carboxylase (PC) pathway for oxidative phosphorylation. [2-13C]pyruvate can directly assess the tricarboxylic acid (TCA) cycle by detecting [5-13C]glutamate production. Preliminary data demonstrated increased lactate and decreased HCO3- (bicarbonate) production from hyperpolarized [1-13C]pyruvate in a rat TBI model and acute TBI patients, however, the role of [13C]HCO3- as a TCA cycle marker needs further verification due to the high pyruvate carboxylation. Another key metabolic alteration following TBI is increased acetate oxidation in astrocytes, playing a neuro-protective role. The increased acetate metabolism tightly interacts with pyruvate metabolism, and thus, should be considered together when interpreting [13C]pyruvate metabolism. The fundamental goal of this project is to understand how TBI influences the in vivo cellular metabolism in the brain using hyperpolarized 13C MRSI as a step towards personalizing therapy for TBI patients. In this project, a comprehensive analysis of TBI metabolism will be performed using a rat TBI model by comparing the in vivo imaging results with ex vivo tissue analysis. First, we will develop hyperpolarized [2-13C]pyruvate as a probe to directly measure the altered TCA cycle activity in TBI (aim 1). Second, we will assess the contribution of increased acetate metabolism to pyruvate oxidation in a rat TBI model (aim 2). The longitudinal in vivo imaging data (aims 1&2) will be validated by cross-sectional ex vivo NMR isotopomer analysis of freeze-clamped brain tissues. Finally, we will translate the technique to assess metabolic changes in acute mild TBI patients (aim 3).
Project 6: HP 13C MRI Technology for the Assessment of Liver Disease
PI: Cornelius von Morze PhD, Washington University, St. Louis
Project Description: This ongoing CP is led by Dr. von Morze who was a trainee at the UCSF HMTRC prior to becoming a faculty member at Washington University in St. Louis.
Significance: To investigate HP 13C MRI technology for the assessment of unmet clinical needs including non-alcoholic fatty liver disease (NAFLD) and other important unmet clinical needs adding HP stable-isotope MR to ongoing PET radionuclide studies. Non-alcoholic fatty liver disease (NAFLD) is a highly prevalent condition characterized by ectopic hepatic lipid accumulation in association with obesity and type 2 diabetes. A progressive derangement of hepatic energy metabolism underlies the progression of simple fatty liver to non-alcoholic steatohepatitis (NASH), a condition that carries high risk for progression to critical liver disease states. Unfortunately, the current method for monitoring NAFLD is liver biopsy, which has major limitations especially its high degree of invasiveness. Hyperpolarized 13C magnetic resonance imaging (MRI) is an emerging molecular imaging modality with a unique capability to access intermediary energy metabolism in a non-invasive manner, which is currently undergoing translation into human studies at several sites internationally in studies of cancer and cardiovascular disease. The goal of this new R01 project is to investigate a new application of this new molecular imaging modality to the assessment of NAFLD. The association between detected changes in hyperpolarized 13C MRI and progression of NAFLD will first be investigated in a preclinical model of the progression of NAFLD to NASH (Aim 1), specifically Zucker diabetic fatty (ZDF) rats fed a high fat diet. Two promising hyperpolarized probes of hepatic energy metabolism, [1-13C]pyruvate and [2-13C]dihydroxyacetone, will be applied to these studies. These new hyperpolarized markers will be compared against gold standard measures of NAFLD derived from pathology, as well as biochemical assays of hepatic energy state and oxidative stress. Next, novel MRI methods will be developed to enable clinical translation of hyperpolarized 13C imaging of the liver (Aim 2). The proposed approach aims to enable dynamic free-breathing imaging of the entire liver by accelerated echo planar imaging (EPI) acquisition with sixteen-channel parallel imaging. To address the key challenge of array sensitivity calibration, a highly novel approach to parallel imaging will be deployed based on continuous tracking of receiver coil positions using an integrated manganese-55 MRI based fiducial marker system. Finally, initial clinical hyperpolarized [1-13C]pyruvate MRI will be performed in normal subjects and patients with simple steatosis and NASH, with comparison to liver biopsy (Aim 3). By the end of this new five-year R01 Research Project Grant, we aim to deliver a valuable new clinical tool for non-invasively assessing the progression of NAFLD based on associated metabolic changes measured using hyperpolarized 13C MRI. The overall goal of this project is to investigate the new application of hyperpolarized 13C MRI technology to the assessment of non-alcoholic fatty liver disease (NAFLD). By addressing important limitations of the “gold standard” method of liver biopsy (particularly invasiveness), this project addresses an important unmet clinical need and therefore has the potential to make a significant impact on the clinical management of liver disease.
Project 7: Improved Methods for Perfusion Imaging with HP Carbon-13 MRI
PI: Aaron Grant PhD, Harvard University
Project Description: This new CP is designed to develop robust new methods for perfusion imaging by making use of hyperpolarized carbon-13 labeled perfusion tracers including freely diffusible ones.
Significance: Disorders of perfusion underlie the majority of the causes of mortality and disability in the developed world. Ischemic disorders, including myocardial infarction, stroke, and pulmonary embolism are obvious manifestations of pathologic perfusion. Perfusion also plays a major role in many cancers. Clearly, methods for high quality perfusion imaging are an important priority for radiologic evaluation of patients. Unfortunately, the resolution, quality, and accuracy of perfusion imaging is still far inferior to the clinical and research needs. Techniques based on arterial spin labeling (ASL) are limited by signal strength and by the short relaxation times of labeled protons. Methods that make use of gadolinium contrast agents face challenges arising from the complex relationship between the underlying perfusion and the resulting image dynamics, in addition to growing safety concerns. This project proposes to develop robust new methods for perfusion imaging by making use of a hyperpolarized carbon-13 labeled perfusion tracer that is freely diffusible in tissue. The tracer has long T1 and T2 relaxation times and long residence times in tissue, and can be imaged with essentially no endogenous background. Consequently, the temporal dynamics of this agent can be monitored using robust imaging techniques with high signal strength. This CP aims to develop methods for measuring local blood flow using dynamic imaging techniques. Hyperpolarized perfusion imaging will be compared against ASL techniques, and both methods will be validated against microsphere perfusion quantitation as a gold standard to assess the accuracy and reproducibility of perfusion imaging with diffusible polarized tracers. Methods for imaging the flow of blood in tissue are critical tools for diagnosis and treatment monitoring in many leading causes of death and disability. Existing methods for blood flow imaging are complex and sometimes unreliable. This research is aimed at developing more robust methods for blood flow imaging, and comparing these new methods to existing techniques to assess their relative performance.
Project 8: HP C-13 MRI Cancer Preclinical and Clinical Research at the NCI
PIs: Amy Leblanc DVM & Murali Cherukuri PhD, NCI Molecular Imaging Group
Project Description: Preclinical and Translation for first NCI human studies in Brain Tumors, Renal, and Prostate Cancer. This ongoing collaboration aims to benefit cancer research at the NCI by disseminating HMTRC preclinical and human HP C-13 technology with training, on-site visits, and collaborative development of 13C-alpha-ketoglutarate as a new biomarker for IDH mutations as supported by the NCI Experimental Therapeutics (NExT) Program. This CP leverages and is funded through the Comparative Oncology Program (COP) laboratory hat is a resource for the development of assays and reagents in support of preclinical drug development and the subsequent study of anti-cancer drugs. The overriding mission of the COP laboratory is to leverage existing knowledge, expertise, techniques and reagents for the study of metastasis biology. Expansion of the existing reagent/assay platforms into other types of cancer e.g. brain tumors could be a consideration as the program continues to develop. The integration of the COP's clinical trial infrastructure with customized assay development provides opportunities to improve the understanding of cancer growth and response to treatment, translate biological concepts toward clinical application, and ultimately provide new therapeutic options to patients. The work of the COP laboratory on metabolomics and metabolic imaging has expanded via collaboration with Dr. Peter Choyke/MIP and aims to interrogate metabolic differences quantitatively using a variety of techniques such as Seahorse extracellular flux analysis and 13C-pyruvate hyperpolarized MRI, for in vitro and in vivo investigations, respectively. Drs. Choyke and Cherukuri are conducting metabolomics studies and metabolic imaging with a focus on hyperpolarized MR and isotope-resolved analysis of clinical samples and patient-based studies. The Mass Spectrometry and Sample Preparation Core Facility is geared toward targeted, ultra-high resolution stable isotope-resolved metabolomics as well as careful clinical tissue sample extraction and preparation methodologies. The first mass spectrometer to be installed will be a 1,000,000 resolving power Thermo Orbitrap Fusion Lumos spectrometer equipped with a Dionex IC-6000 Ion Chromatograph and an Advion TriVersa Nanomate direct infusion electrospray ionization modules, which will be used to develop workflows tailored for targeted quantitation of approximately 120 cellular metabolites. Renovation of the space housing the Clinical NMR Metabolomics Facility was completed in November of FY20, and delivery and installation of a 700MHz Bruker NMR magnet, triple inverse resonance cryoprobe and high-capacity chilled autosampler was completed in May 2020. Data collection and method development for NMR analysis of metabolite extracts from clinical tissue specimens obtained during surgical and biopsy procedures in the NIH Clinical Center are currently underway. Finally, procurement of a custom 13C body coil and consumables for validation and preparation of 13C-pyruvate doses for future clinical 13C-hyperpolarized metabolic imaging are taking place and will benefit greatly from the technology development, training and dissemination that would be provided through the HMTRC.
Project 9: Prostate Cancer HP C-13 MR Research in Patients and Preclinical Models
PIs: Rahul Aggarwal MD & Donna Peehl PhD, UCSF Oncology, Urology, Radiology
Project Description: This is an ongoing CP at UCSF that is expanding in novel directions with new NIH funding. Also Dr. Peehl’s basic science investigations in a U24 co-clinical trial will investigate the metabolic reprogramming underlying the clinical research findings.
Significance: Prostate cancer is a major US health concern with over 160,000 new cases and 30,000 deaths per year. More than 90% of patients with advanced disease have bone metastases with a median survival of a year. Patients with metastatic bone disease suffer from bone pain, fractures, hypercalcemia, cytopenias, and ultimately death. The goal of this early phase “feasibility” imaging “biomarker-driven” trial is to investigate novel 3D hyperpolarized (HP) 13C-pyruvate MRI techniques to quantitatively measure lactate dehydrogenase (LDH) catalyzed pyruvate- to-lactate (kPL) conversion rates in prostate cancer bone metastases to enable early and rapid metabolic response monitoring to treatment response and development of therapeutic resistance, as well assess on-target treatment effects for new targeted drug development, thus addressing current unmet clinical needs. This clinical trial is required because current imaging modalities for bone metastases are inadequate for quantifying response to therapeutic interventions. This can result in significant delays in determining treatment effectiveness, and subjecting patients to prolonged periods of side-effects of ineffective therapies, too often causing excess morbidity without benefit. Hyperpolarized (HP) 13C-pyruvate MRI is a safe, non-radioactive, quantitative MR stable-isotope imaging approach that can provide early, real-time metabolic response monitoring of prostate cancer bone metastases. Added to conventional mpMRI exams, the rapid 2 minute HP MRI measurement of pyruvate-to-lactate conversion rate, kPL, a potentially valuable “biomarker”, reflects changes in metabolic reprogramming and early response to targeted therapies (e.g. AR, MYC inhibitors). While PSMA-PET improves detection of metastatic disease, PSMA expression is not directly affected by targeted therapies including androgen pathway inhibitors and novel MYC-targeted therapies in clinical trial evaluation, and therefore is not able to reliably capture bone metastasis response at early time points. HP 13C-pyruvate MR studies have demonstrated that MYC-mediated increased HP 13C pyruvate-to-lactate metabolic conversion rate, kPL, is associated with key oncogenomic alterations that occur with the progression to advanced prostate cancer and decreased in response to treatment. This new biomarker-driven trial is designed to apply new HP 13C-pyruvate MRI technology for monitoring androgen-receptor and MYC targeted drug therapies with the goal of investigating kPL as a quantitative in vivo marker to measure metabolic changes with treatment in patients with bone-tropic advanced prostate cancer. The Co-Clinical Prostate Cancer Imaging Research Program project led by Dr. Peehl focuses on the establishment of preclinical quantitative HP 13C MRI protocols, data analyses tools, and correlative biology data allowing for a consensus on how quantitative HP 13C MRI can be used in co-clinical imaging trials to improve the assessment of therapeutic response and resistance. While this project focuses on advanced prostate cancer, these new quantitative metabolic imaging techniques could ultimately benefit the clinical management of other cancers and diseases
Project 10: Investigating Disrupted Metabolism in Alzheimer's Disease by HP C-13 MRI
PI: Ken Nakamura MD/PhD, UCSF Neurology
Project Description: This new CP aims to probe disrupted cerebral energy metabolism in Alzheimer's disease (122,019 deaths/yr in US) by applying HP C-13 MR combining the extensive expertise and resources of the UCSF Alzheimer's disease (AD) research program with the unique facilities, equipment and experienced personnel in the HMTRC.
Significance: Alzheimer's disease (AD) is characterized by early and prominent changes in glucose metabolism and mitochondrial respiratory enzymes. In AD patients and asymptomatic ApoE4 carriers regional brain glucose uptake is decreased. However, we do not understand why these changes occur, how they contribute to neurodegeneration, or whether they can be targeted therapeutically. To gain insight into how AD proteins change glucose metabolism, we developed innovative assays to measure glucose metabolism in cellular and mouse models of AD. With this approach, we can test our central hypothesis that, in AD, mutant (mut)-amyloid precursor protein (mutAPP) and ApoE4 inhibit distinct steps in glucose metabolism and the respiratory chain, resulting in energy failure and impaired pentose phosphate pathway (PPP) function. The overall objective of our proposed study is to determine how mutAPP and ApoE4 alter glucose metabolism in neurons. We will accomplish this objective in two specific aims: (1) We will use live imaging and mass spectrometry to measure metabolic fluxes, and determine how mutAPP and ApoE4 disrupt glucose metabolism and bioenergetics in cultured neurons. (2) We will use metabolic imaging to determine how mutAPP and ApoE4 converge to disrupt glucose metabolism with age in mice. Overall, these studies will i) provide important insights into how mutAPP and ApoE4 converge to disrupt glucose metabolism and energy production, ii) develop clinically translatable metabolic imaging approaches for AD, and iii) form the basis for new therapeutic strategies to normalize neuronal metabolism in AD. While Alzheimer's disease (AD) is known to be characterized by early and prominent changes in glucose metabolism and mitochondrial respiratory enzymes, we do not know why these changes occur or how they contribute to neurodegeneration. This CP aims to study how key proteins that cause AD disrupt glucose metabolism and bioenergetics in neurons using HP C-13 MRI. These studies will help us understand how glucose metabolism is disrupted in AD and how this process might be targeted therapeutically.
Interested in a starting New Collaborative Project? Click here to learn more.
Service Projects
Project 1: Translational HP Carbon-13 MR Studies of Cancer Patients
PI: Dirk Mayer PhD, Maryland University
Project Goal: Dr. Dirk Mayer has extensive experience in HP MRI with onsite training and dissemination from this NCBIB when he was at Stanford University and now via remote dissemination at the University of Maryland (but with visits to UCSF). This SP will focus on upcoming Human Bladder, Prostate Cancer and Brain HP MRI studies that have been designed to address important unmet needs in the characterization of cancer aggressiveness and response to therapy. These studies were planned with substantial input from the HMTRC by direct hands-on meetings at UCSF, email exchanges, website, on-line file sharing, and through the bi-weekly webconferences.
Project 2: Elucidating the Role of Trop2 in Prostate Cancer
PI: Tanya Stoyanova PhD, Stanford University
Project Goal: This project aims to perform analysis of changes in steady state metabolites and metabolic flux using multiple HP MR probes induced by Trop2 and explore the ability of HP MRI at UCSF via TRD2 to image trans-differentiation of adenocarcinoma to small cell neuroendocrine prostate cancer based on these metabolic changes. This NIH R37 grant at Stanford University focuses on the role of Trop2 in castration-resistant prostate cancer (CRPC) and small cell neuroendocrine prostate cancer (SCNC). Trop2 is over-expressed in these cancers and enhances tumor growth, invasion, migration, and trans-differentiation of adenocarcinoma to neuroendocrine cancer (Hsu et al. 117:2032 2020). The aims of the R37 project are to delineate the mechanisms by which Trop2 causes the emergence of CRPC and SCNC, promotes tumor growth, and increases metastases. Functional enrichment analysis of Trop2-regulated proteins from whole proteomic profiling revealed that 9 of the top 16 biological processes from gene ontology (GO) involved metabolism. However, the R37 grant does not include studies to investigate and define the metabolic changes that occur in response to overexpression or downregulation of Trop2 in prostate cancer (PCa). This Service Project will enable analysis of changes in steady state metabolites and metabolic flux induced by Trop2 and explore the ability of HP MRI to image trans-differentiation of adenocarcinoma to SCNC based on these metabolic changes. Approach: (1) To characterize metabolic shifts occurring upon Trop2-mediated transition of adenocarcinoma PCa to SCNC, and its reversal, the isogenic PCa cell lines LNCaP, LNCaP-Trop2-OV and LNCaP-Trop2-OV-shTrop2 will be labeled with [U-13C]glucose and [U-13C]glutamine and metabolic flux through glycolysis, the tricarboxylic acid (TCA) cycle, glutaminolysis, and glutathione synthesis will be analyzed by nuclear magnetic resonance (NMR) spectroscopy. (2) Slurries of each of the cell lines will be placed in a bioreactor and HP 13C NMR spectroscopy will be performed. (3) If studies in (1) and (2) identify specific metabolic changes associated with adenocarcinoma versus SCNC phenotypes, the cell lines will be implanted in mice and HP 13C magnetic resonance imaging (MRI) with the appropriate substrate(s) will be performed on the xenografts.
Project 3: Predictive Imaging-Derived Signatures of Cancer
PI: Charles Manning PhD, UT MDACC, Houston, TX
Project Goal: The goal of this new SP research is to develop imaging tools to predict tumor response to glutamine metabolism-targeted therapies using novel PET imaging agents. This service project will apply metabolic studies using multiple HP MR metabolic probes (TRD2) from the HMTRC to provide insight into the metabolic changes associated with treatment that lead to the observed PET biomarkers of therapeutic response. Precision cancer medicine, which seeks to exploit unique cellular, molecular and genetic characteristics of individual tumors to optimize treatment, remains a critically unmet need. Despite advances in biomarker technologies that yield high-quality cellular and genomic data, critical gaps remain to consistently match patients with cancer and ideal therapies. While `predictive' genomic assays based on RNA and DNA are now commonplace, current methods largely ignore tumor phenotypes differentiable by quantitative imaging. The overarching vision for this project is to create and evaluate a suite of quantitative imaging tools that facilitate the discovery of novel, predictive imaging-derived gene expression signatures; such signatures can be deployed by the greater oncology community to improve the personalization of cancer treatment.
Project 4: Monitoring PI3K-Inhibitor Treatment of Advanced Breast Cancer with HP C-13 MR
PI: Gerburg Wulf MD/PhD, Harvard University
Project Goal: This funded project addresses the problem of advanced, PIK3CA-mutant breast cancer. We propose a role for PI3K-inhibitors in this disease at a stage when the disease no longer responds to endocrine manipulation, cdk4/6- or mTOR-inhibition and when patients typically receive palliative chemotherapy. We propose a role for next-generation PI3K-inhibitors at later stages of the disease, when PIK3CA-mutant tumors have developed endocrine, cdk4/6i and mTORi resistance. In three independent aims our goal is to develop treatment strategies for women with endocrine-resistant PIK3CA mutant breast cancer. Metabolic imaging will be used to advance that goal. Understanding the distinct anti-metabolic function of PI3Ki that are active in heavily pretreated disease will allow us to develop combination treatments and administration schedules that can be translated into clinical trials concepts. This new SP project is designed to determine in vivo if the metabolic changes induced by PI3K-inhibition are predictive of cancer treatment responses. It aims to visualize lower glycolysis directly in tumors in vivo using NMR detection of 13C-pyruvate metabolism. Simultaneous studies of the flux of 13C-glucose and 13C[1]-pyruvate will be used to pinpoint mechanisms of resistance. Another aim is to develop treatment combinations that may improve the outcomes for women with metastatic BC. Given the vulnerability of BC cells in the early post-mitotic phase, this project proposes to explore PI3K-inhibition following infusion of taxanes or vinca alkaloids in mouse models of PIK3CA-mutant endocrine-resistant BC.
Project 5: Development of Photo-Hyperpolarizable Nanodiamond Based 13C Contrast Agents
PI: Ashok Ajoy PhD, UC Berkeley
Project Goal: The purpose of this technology development project is to develop photo-hyperpolarizable nanodiamond based 13C contrast agents and decoupling schemes to achieve a dramatic – at least three to four orders of magnitude – boost in 13C NMR signal to enable high resolution deep tissue MRI including affordable low field MRI scanners with initial testing in animals at the HMTRC. Multimodal imaging - the ability to acquire images of an object through more than one imaging mode simultaneously has opened additional perspectives in areas ranging from astronomy to medicine. This project is focused on combining optical and magnetic resonance (MR) imaging in such a "dual" imaging mode. They are attractive in combination because they offer complementary advantages of resolution and speed, especially in the context of imaging in scattering environments. Our approach relies on a specific material platform, microdiamond particles hosting nitrogen vacancy (NV) defect centers that fluoresce brightly under optical excitation and simultaneously "hyperpolarize" lattice nuclei, making them hyper-intense under MR imaging.
Project 6: Hyperpolarized Carbon-13 Metabolic MRI of the Human Heart
PI: Roselle Abraham MD, UCSF
Project Goal: Alterations in cardiac metabolism are implicated in a broad range of heart diseases, including cardiomyopathies, ischemia, and early heart failure. The project proposes to develop a novel imaging modality, hyperpolarized 13C MRI, for metabolic imaging to assess heart disease. Hyperpolarized 13C MRI has transformative potential by providing unique quantifications of metabolic fluxes for earlier and more precise diagnoses, improving understanding of pathogenesis, and rapid assessments of treatment response.
Project 7: Imaging and Biomarkers for Early Detection of Aggressive Prostate Cancer
PIs: Alan Pollack MD/PhD & Radka Stoyanova PhD, Miami University
Project Goal: Oversampling and overdiagnosis of prostate cancer are significant management and cost issues that burden our health care system and the individual at risk with unnecessary biopsies and potential complications. The proposed studies will validate recent advances in quantitative prostate multiparametric MRI (mpMRI) techniques, blood biomarkers of aggressive prostate cancer and radiogenomics that relate to increased aggressive cancer risk by our group and collaborators. The overarching goal is to increase the negative predictive value (NPV) for significant prostate cancer and consequently reduce unnecessary biopsies. This new SP prostate cancer research combines quantitative imaging features to validate/extend that the developed mpMRI based habitat risk scoring system is superior to the more standard PIRADSv2 in identifying significant cancers in the prostate prior to guided biopsy and through this SP will investigate the addition of HP data collected at UCSF.
Project 8: Hyperpolarization Assisted Screening of Protein-Ligand Interactions in Live Cells
PI: Christian Hilty PhD, Texas A&M University
Project Goal: Protein-ligand interactions play a pivotal role in fundamental biological processes including cellular signaling and regulation. Experimental screening for interactions with drug candidate compounds and fragments further represents an indispensable step in drug discovery. Traditional methods for determining such interactions often require highly purified proteins, which in particular are not always available in the case of membrane proteins. The same limitations exist for the characterization of functional complexes formed by recruitment of multiple constituents on a membrane or within a cell. Notwithstanding, 60% of current drugs target membrane proteins. Here, a method will be developed for determining interactions between small molecules of arbitrary type, directly with receptors or other components located on the surface or within a cell. Nuclear magnetic resonance (NMR) signals of ligands will be enhanced by several orders of magnitude using dissolution dynamic nuclear polarization (D-DNP). Transmembrane proteins among other functions are responsible for signal transmission between cells. A large fraction of drugs being currently marketed or developed target membrane standing receptors, including the G- protein coupled receptors. This project aims to develop a method that allows the identification of binding and determination of binding epitope structure of receptors in the natural environment in a cell, which would greatly facilitate the drug discovery process. This new SP project aims to develop a method that allows the identification of binding and determination of binding epitope structure of receptors in the natural environment in a cell, which would greatly facilitate the drug discovery process.
Project 9: Translating HP 13C Metabolic MRI to Predict Renal Tumor Aggressiveness
PI: Jane Wang MD, UCSF Radiology
Project Goal: This study will apply hyperpolarized 13C metabolic MRI to renal tumors to address an unmet need for noninvasive predictors of tumor aggressiveness. This project aims to clinically translate hyperpolarized (HP) 13C pyruvate MRI as an innovative metabolic imaging approach for noninvasive prediction of renal tumor aggressiveness, an unmet clinical need. This project is motivated by the rising incidence of renal tumors, largely due to the increased utilization of imaging with incidental discovery of many localized tumors. These include both benign renal tumors and malignant renal cell carcinomas (RCCs). Current imaging or biopsies cannot reliably differentiate between benign tumors, low grade RCCs, and high grade RCCs. The diagnostic ambiguity has led to an overdiagnosis of many indolent tumors which are unnecessarily treated by surgery with surgical risks, and importantly, increased risk of chronic kidney disease and associated cardiovascular disease. Notably, the increased detection of RCCs has not translated into a decrease in cancer specific death. Therefore, there is a significant unmet need for novel imaging markers that can improve the risk stratification of localized renal tumors to guide patient management. HP 13C MRI is an emerging imaging technology that allows real-time pathway-specific investigation of metabolic processes that were previously inaccessible by imaging.
Project 10: HP MR Metabolic Imaging of Multiple Sclerosis
PIs: Myriam Chaumeil PhD & Caroline Guglielmetti PhD, UCSF Physical Therapy
Project Goal: Multiple sclerosis (MS) is a multifaceted neurological disease and one of the most common causes of disability in young adults. The major hallmark of MS is an uncontrolled immune response that drives demyelination, resulting in continuously worsening cognitive impairments, and eventually leading to death. Upon clinical diagnosis of MS, numerous clinical evaluations and MR Imaging (MRI) sessions are required to assess symptoms and/or presence of lesions. Even then, prediction of outcome and choice of treatment remain a challenge for each patient. Given the role of inflammation in MS, an imaging method that could detect immune response could improve patient management and subsequent individualized therapeutic approaches. In MS lesions, mononuclear phagocytes (MPs, macrophages/microglia) are the most abundant immune cells, and drive demyelination and cell death. Interestingly, to sustain high proliferation rates, these MPs have switched from quiescent to pro-inflammatory (M1-polarized) activated state and show increased lactate production linked to increased pyruvate dehydrogenase kinase 1 (PDK1). The goal of this study is to test the hypothesis that MPs activation and M1/M2 status can be detected in MS lesions in vivo using hyperpolarized 13C MR Spectroscopic Imaging (HP 13C MRSI) and that such metabolic imaging can improve evaluation of MS progression and treatment response. This project aims to improve MR metabolic preclinical imaging of Multiple Sclerosis by applying HP 13C MRI in animal models to understand the underlying biochemical processes in this disease.
Interested in a starting New Service Project? Click here to learn more.